
By Bob Goemans

There's no doubt this subject matter has and continues to generate much interest. And that some of the terms used to describe different type beds and their functions, e.g., anoxic, anaerobic, hypoxic, dysaerobic, diffusion, and bioturbation, have been interpreted differently by many aquarists. This has all led to differing opinions as to the value or the understanding associated with certain processes in different type sandbeds. And the largest diversity lies between those favoring deep sandbeds (DSB) directly on the aquarium bottom and those preferring the Jaubert plenum method. Part of this discord also includes those who prefer mud-like sediments or courser-grained material. We find this quite disconcerting since all of these methods can produce reasonable results when associated with sensible objectives. Therefore, our goal here is not to single out either method as the better, but to explain their differences in a clear and understandable fashion.
This goal first begins with trying to establish a clear picture of the different terms being used, then an explanation of each method, and then a series of positions/facts that pertain to each method. As a whole, we think the reader will find it enlightening and they can then follow the path that best suits them and the animals in their aquariums.
To begin, the phrase 'biological filtration' is commonly used throughout our industry, however, we must state our dislike for this term. 'Filtration' is a process or means in which something is removed or exported. It is filtered out so to speak. On the other hand, bacteria use a process called 'metabolism' to accomplish environmental changes by transforming elements and compounds into something different. Without a doubt various microbial processes are the foundation of every aquarium system. Healthy longevity of which is based upon how well they (bacteria) function. How well they function is in turn dependent upon your knowledge of their existence and their requirements. You could say they are the heart and soul of the system. For all practical purposes aquarists will continue to use this term, but hopefully your gray matter has been tweaked somewhat.
When it comes to words such as anoxic, anaerobic, and hypoxic, each discipline tends to emphasize its own set of properties, partly with different definitions, nomenclature, and standard methods for their measurements. The key is to define their use up front so everyone remains on a level playing field when reading their work and we have done just that in our CD book and other writings. We have used the word 'oxic' and/or 'aerobic' to represent oxygen rich areas. The word 'anoxic' instead of the little recognized word 'dysaerobic' or the mostly medical term 'hypoxic' to represent areas where a little oxygen is present. And, which we believe to contain approximately .5 to 2.0 mg/l and where destructive denitrification occurs. And the well-known word 'anaerobic' to represent areas that contain less than the anoxic condition noted above, and where the ammonification form of denitrification occurs. Our definitions were decided upon to satisfy the general audience, not the scientific world, and simply denote the reduction of oxygen from oxic to anoxic and then to anaerobic conditions. (See the sketch below.)
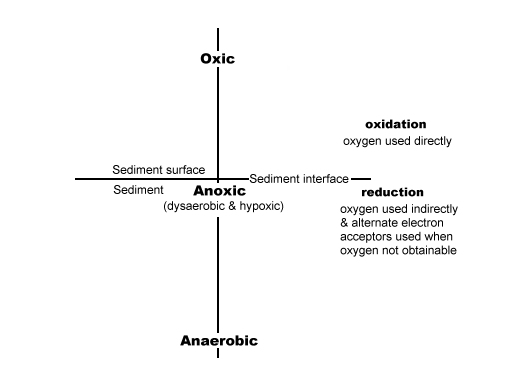
Lets now review the meaning of the word's 'diffusion' and 'bioturbation.' Also, the words 'porewater' and 'oxygen' as they need some explanation if these articles are to be useful.
Porewater is the minute water-filled area around and between sand particles. Bacteria adhere to the particles and utilize this adjacent area, which is an active region of diffusion for all elemental components of energy flow and nutrient cycling. And, the smaller the sediment particle, the closer each particle is to each other, thereby reducing the amount of useful solution filled space between them.
Diffusion is the all-encompassing way nature moves molecules from one place to another. It could also be said it's the movement of solutes in a fluid down a gradient from an area of higher concentration to an area of lower concentration. At times, other forces are aiding - solutions are stirred, the winds blow causing wave action, the oceans have currents, and even our own body cells have internal flows. Nonetheless, diffusion is the fundamental transport process. It encompasses how molecules collide and react; how food molecules approach cells, and how wastes leave local environments. It's a very dynamic process that helps many biological functions, and physical factors, e.g., temperature, density, and pressure, also play dynamic roles.
Both in the wild and aquariums, diffusion to one degree or another exists. Yet there still remains not enough evidence or research data to make statements set in stone as to all its interactions. For example, permeability and advection affect diffusion in major mechanisms which in turn determine the action and fate of not only solutes (stuff dissolved in the water), but also the distribution and fate of the microbial mediators themselves.
It is also important to understand the term 'permeability,' which relates to the physical shape and distribution of the sediment (sand). Fine-grained sediments have low permeabilities, thus restricting possible porewater flow and associated dynamics. Molecular diffusion and possibly animal irrigation dominate solute transport and consequently, oxygen penetration. Fine sediments sometimes have high organic matter loading. If so, they tend to go anoxic/anaerobic at very shallow depths - between a couple of centimeters to a few millimeters, such as in many coastal areas. That's because diffusion cannot effectively supply the oxygen demanded by organic matter decay.
Coarser sediments, i.e., above .5 mm, have higher, more useful permeabilities. In the wild, water flow around, near and at these grain size sediments (advection) can penetrate somewhat and dominate over diffusion as the transport process for solutes in porewater close to the bulk water-sediment interface. And, with higher current flows the supply of oxygen for organic matter decomposition can easily be met by porewater advection and the sediments can stay oxic (and low in carbon) even if they have the same input of organic matter as a muddy sediment.
Regrettably the concept of advective transport is less likely to aid diffusion in aquarium substrates, especially where very fine grain material is used. We have often heard that the best sand is very fine sand. And that's because when there are more sand grains there's greater total surface area, therefore more microbes. Even though that's true, it's of no value when one thinks of overall system balance. Small grains pack together very tightly and the space between them, porewater, is greatly reduced. When that happens, anoxic conditions (defined in previous writings and above) are restricted to an extremely shallow depth just below the upper oxic area. Important microbial processes, i.e., mineralization, nitrification and destructive denitrification all have greatly reduced volumes because there is less penetration of oxygen overall without a means to infuse oxygen. The remainder of the bed becomes anaerobic (also defined in previous writings and above) and is where Ammonification, a form of denitrification that only reduces nitrate back to ammonium becomes prevalent. Basically it's more microbes but not the right 'class' of microbes such as facultative anaerobic heterotrophs instead of obligate anaerobic heterotrophs. And in such cases, bioturbation, i.e., increased infauna irrigation and transport, become a more dependent way of life than diffusion. Yet, it's fair to say that because most aquarium beds use a course sand grain, i.e., above .5 mm, most properly maintained sandbeds (probably over 90%), fall into the category of utilizing diffusion processes to supply most of its bioactivity.
Bioturbation is the disturbing and altering of a surface and subsurface of substrates by the physical activity of organisms such as worms, crustaceans, and foraminiferans (infauna). In the wild it generally dominates in fine sand and mud-like substrates and in deeper beds where diffusion is greatly limited. It's important to note that relatively simple alterations to the transport-reaction structure, typical of the bioturbated zone, can result in dramatic changes. In fact, the mechanics of bioturbation can alternately provide another means of transport of bio-geochemical constituents. Whether these effects are good or not for the equilibrium of the aquarium system depends on various factors. For example, infauna tunneling may help release of ammonium or cause increased nitrification rather than destructive nitrate dissimulation because of elevated oxygen levels, or facilitate the release of phosphate into the bulk water. These concomitant factors stress the need to view bioturbation as processes that do not replace diffusion.
Furthermore, balance between coupled redox reactions such as nitrification-denitrification, and acid-based sensitive reactions such as carbonate precipitation-dissolution can be completely altered by small changes in burrow size and spacing patterns. To conceptualize and quantify complex solute distributions and fluxes within the bioturbated zone a variety of transport-reaction models have been utilized, e.g., enhanced diffusion, enhanced advection, internal source-sink or non-local transport, and microenvironment or analog geometries to the burrowed zone. Each model category has advantages and disadvantages resulting from a best-fit application. Each is appropriate for particular circumstances, particular use, and explanation of basic phenomena. However, they shouldn't be over generalized for the purpose of answering questions relating to the dynamics of sandbeds in the majority of aquariums.
Moreover, despite demonstrable effects there has been relatively little effort to relate biogenic sedimentary structure ranging from the integrated population or small-scale individual levels to that of communities, e.g., seldom evaluating faunal size, spacing distributions, or activity patterns. Furthermore, geochemical studies typically emphasize overall fluxes such as provided by homogenous box models and often ignore controlling details and fundamental dynamic relations within deposits. Therefore, interactions between reactions, organic matter decomposition pathways, transport regime, and micro-scale properties such as pore-filling secretions remain a largely unexplored area of both modern sedimentary bio-geochemistry and the study of the evolution of surficial bio-geochemical cycles. The same is true of complex geometric structures formed by individual burrows or burrow groupings. To simply parameterize complex model processes could lead to inappropriate and misleading applications in some closed systems.
Another area that sometimes causes confusion when reading articles from different sources is with the use of the word 'oxygen.' It's sometimes written as dissolved oxygen or molecular oxygen, and sometimes simply as oxygen. Oxygen exists either in the gaseous form (molecular oxygen (O2)) or in water as dissolved oxygen. When relating to sandbeds, oxic bacteria at the upper most segment of the sediment are the major users of dissolved oxygen (DO), primarily for metabolic needs. DO is also what's measured when one takes a water sample from the aquarium for the purpose of testing its oxygen level. As for its presence in the upper most portion of the sediment, even that is a varying subject because of the interaction between autotrophs and heterotrophs and diurnal cycles (oxygen used versus oxygen produced at which time of day). When traveling downward into the sediment, the word 'oxygen' is mostly associated with the complex reactions taking place. And when it comes to our sandbeds, the element oxygen (O) is tied to all kinds of things. However, the real issue is oxygen flux from the benthic boundary layer (BBL) (basically the sandbed surface-bulk water interface area) on down to the anaerobic zone via diffusion, bioturbation, or combination of both.
As for a description of the plenum method, its the placing of a sandbed consisting of sand grains in the size of 1 to 4 mm and approximately at a depth of 4 inches, on a slightly elevated platform on the aquarium bottom. Its primary path of bioactivity is via diffusion, yet some bioturbation also comes into play. As for the DSB, it's usually a deep bed of sand directly on the aquarium bottom. Depending upon grain size and depth, its bioactivity results from some diffusion, yet mostly dependable upon infauna (bioturbation).
Next month, Part II will go into preconceived and actual differences between the two methods along with some insight as to their results. Don't miss it!
References
Aller, R.C. 2000. The Benthic Boundary Layer: Transport Processes and Biogeochemistry, Ed. Bernard P. Boudreau, Bo Barker Jørgensen, Ch. 11. Transport and Reaction in the Bioirrigated Zone, Oxford University Press © 2000
Boudreau B.P., Jørgensen, B.B., 2000. The Benthic Boundary Layer: Transport Processes and Biogeochemistry. Oxford University Press © 2000
Jaubert J., 1989. An integrated nitrifying-denitrifying biological system capable of purifying seawater in a closed circuit aquarium. Bull. Inst. Océanogr. Monaco. 5: 101-106.